Dr Wael Abdelrahman DVM PhD MBA
Introduction
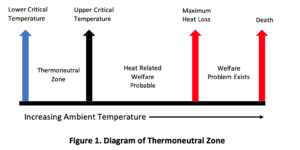
Heat stress in poultry occurs when birds struggle to balance internal heat production with heat loss to their environment. Birds within their thermoneutral zone can regulate body temperature through normal behaviour. However, when the ambient temperature exceeds the upper critical limit, birds must actively lose heat through panting, a natural and non-alarming response at first.
As temperatures continue to rise, the panting rate increases. When heat accumulation surpasses the bird’s maximum capacity for heat dissipation, either in intensity or duration, mortality becomes a real risk (Figure 1).
Importantly, welfare concerns emerge before birds reach this maximum heat load. Stockpersons should remain vigilant and recognize early signs of thermal discomfort.
Changing Climate, Changing Challenges
Modern poultry operations are typically conducted in climate-controlled housing designed to optimize welfare and performance. However, several changes over the past four decades have increased birds’ vulnerability to heat stress:
- Genetic selection for rapid growth and high productivity.
- Altered nutritional strategies and reduced use of medications.
- Increasing frequency of summer temperatures exceeding 30°C globally.
High ambient temperatures, especially when paired with elevated humidity, pose significant risks to both animal welfare and economic performance. This necessitates a re-evaluation of housing, feeding, and management practices to adapt to today’s hotter climate.
Behavioural Signs of Heat Stress
As heat stress develops, birds modify their behaviour to regulate body temperature. Common signs include:
- Distancing from other birds to reduce heat transfer.
- Seeking cooler surfaces.
- Lifting wings to increase heat dissipation.
- Panting and open-mouth breathing.
- Resting to reduce metabolic heat.
- Decreased feed intake.
- Increased water consumption.
- Blood redirected toward the skin for heat loss.
Physiological Consequences
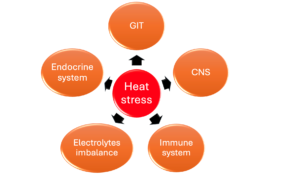
Prolonged heat stress can severely compromise bird health and productivity (Figure 2 and 3):
- Fatigue from excessive panting, negatively affecting welfare.
- Electrolyte imbalances (↓ potassium and phosphate, ↑ sodium and chloride).
- Elevated corticosterone levels (stress hormone).
- Reduced immune competence and greater disease susceptibility.
- Gut integrity disruption, promoting endotoxin leakage and systemic inflammation.
- Depressed feed intake and compromised growth performance.

Mitigation Strategies
Addressing heat stress requires proactive, integrated solutions that consider both management and nutrition:
- Flock and Housing Management
- Lowering stocking density reduces total heat load and improves airflow.
- House design improvements in insulation, ventilation, and orientation can minimize heat accumulation.
- Feed withdrawal during peak heat hours may reduce metabolic heat and mortality risk.
- Cool water availability stimulates intake and counters dehydration.
- Nutritional Adjustments
- Reformulate diets to compensate for reduced feed intake while maintaining nutrient supply.
- Optimize the energy-to-protein ratio.
- Adjust levels of key electrolytes, vitamins, and minerals.
- Incorporate water supplements such as glycine, betaine and plant extracts that support thermoregulation and stress resilience.
One such solution is UtriCool, a precision-formulated water additive developed by UTRIX to help birds cope with high temperatures. UtriCool contains a targeted blend of electrolytes, antioxidants, and natural cooling agents that:
- Support thermoregulation and reduce oxidative stress.
- Improve water intake and hydration status.
- Maintain electrolyte balance during periods of high panting and heat-induced fluid loss.
Conclusion
Heat stress poses a serious threat to both poultry welfare and production efficiency. It requires a strategic approach involving environmental modifications, thoughtful management, and precise nutritional intervention. With the right actions, the negative impact of heat stress can be significantly reduced, ensuring better health, performance, and profitability.
References
- Hall, D. M., Buettner, G. R., Oberley, T. D., Xu, L., Matthes, R. D., & Gisolfi, C. V. (2001). Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. American Journal of Physiology-Heart and Circulatory Physiology, 280(2), H509–H521.
- Wampler, J. L., Kim, K. P., Jaradat, Z. W., Bhunia, A. K. (2004). Heat shock protein 60 acts as a receptor for Listeria adhesion protein on Caco-2 cells. Infection and Immunity, 72(2), 931–936.
- Sakurada, S., & Hales, J. R. (1998). Anatomic distribution of thermoregulatory responses to hyperthermia. Journal of Applied Physiology, 85, 381–386.
- Rivera, L. R., Thacker, M., Furness, J. B. (2011). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Advances in Experimental Medicine and Biology, 704, 49–62.
- Rozenboim, I., Tako, E., Gal-Garber, O., Proudman, J. A., Uni, Z. (2007). The effect of heat stress on ovarian function of laying hens. Poultry Science, 86(8), 1760–1765.
- Teeter, R. G., Smith, M. O., Owens, F. N., Arp, S. C., Sangiah, S., & Breazile, J. E. (1985). Chronic heat stress and respiratory alkalosis: Occurrence and treatment in broiler chicks. Poultry Science, 64(6), 1060–1064.
- Mashaly, M. M., Hendricks III, G. L., Kalama, M. A., Gehad, A. E., Abbas, A. O., & Patterson, P. H. (2004). Effect of heat stress on production parameters and immune responses of commercial laying hens. Poultry Science, 83, 889–894.
- Suk, Y. O., & Washburn, K. W. (1995). Effects of genetic variation on heat tolerance of laying hens: 2. Changes in blood constituents. Poultry Science, 74, 1031–1038.
- DuBose, D. A., Baliko, M., & Czaja, A. J. (2002). Cytokine production and endotoxin sensitivity during experimental heat stress. Shock, 18(6), 541–546.
- Arimura, A., & Shioda, S. (1994). Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine regulator. Peptides, 15(3), 367–372.
- Santos, J., Yang, P. C., Söderholm, J. D., Benjamin, M., & Perdue, M. H. (2000). Role of mast cells in chronic stress-induced colonic epithelial barrier dysfunction in the rat. Gut, 48, 630–636.
- Söderholm, J. D., & Perdue, M. H. (2001). Stress and gastrointestinal tract. II. Stress and intestinal barrier function. American Journal of Physiology-Gastrointestinal and Liver Physiology, 280, G7–G13.
- Wilson, R. M., & Baldwin, D. M. (1999). Effects of stress on mucin release and goblet cell numbers in the rat intestine. Journal of Nutrition, 129, 1062–1067.
- Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X. N., … & Koga, Y. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. Journal of Physiology, 558, 263–275.